IV
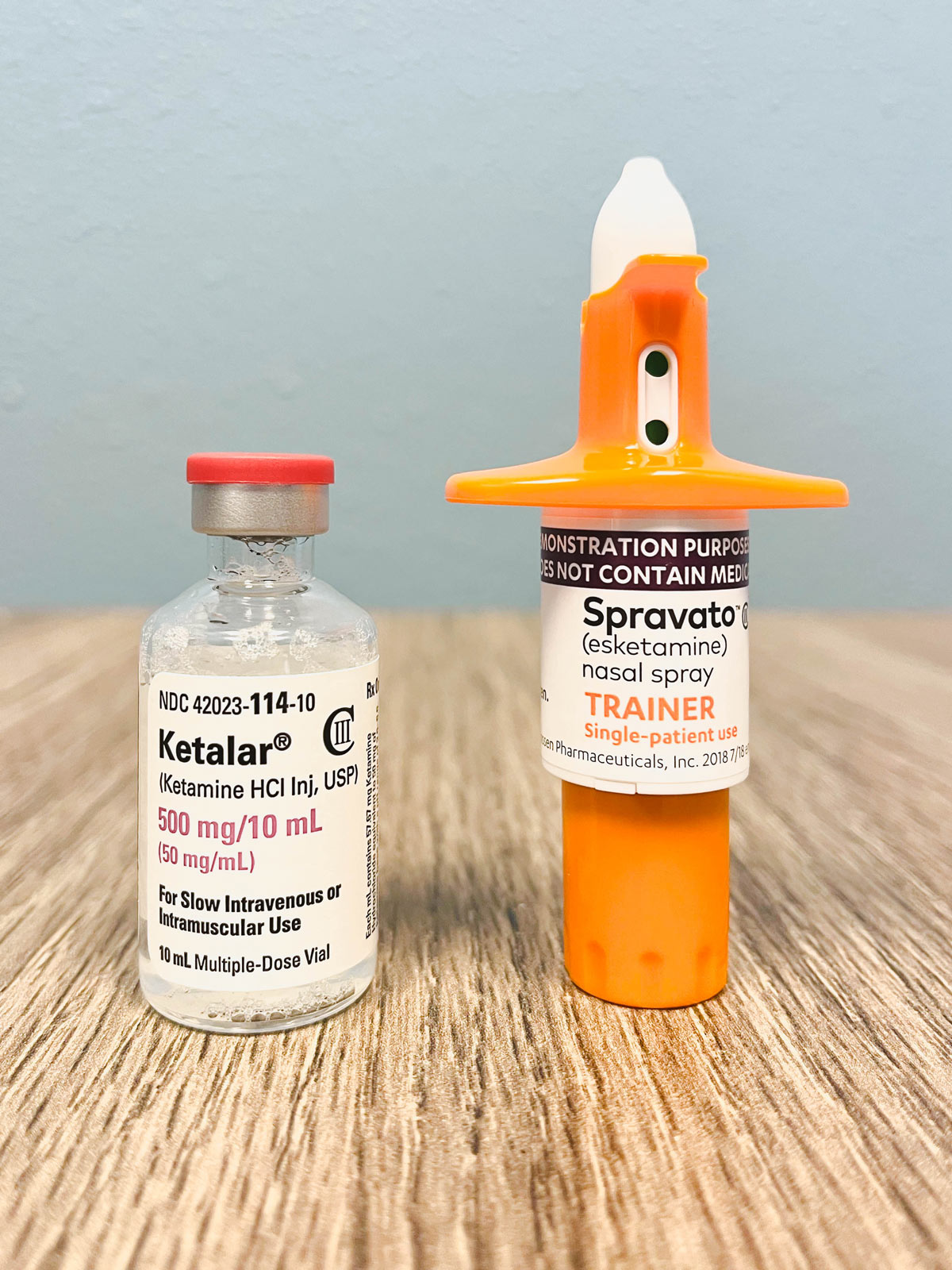
Using ketamine for TRD is considered off-label. “It is estimated that between 40 and 60 percent of all prescriptions written in the United States are for off-label uses.” - James B Riley, Jr & Aaron Basilius, Hematology & Oncology News & Issues, Vol6, No.5. Because its use in TRD is off-label, IV ketamine treatments are a cash pay treatment. If you choose to seek reimbursement from your insurance carrier, we are happy to provide a superbill which will be required by the carrier.
SPRAVATO
Spravato is an FDA approved intranasal treatment of esketamine (a specific form of ketamine) for TRD. The great news about it being FDA approved is that it is insurance reimbursable. Please visit the product website for additional information at spravato.com/trd/about-spravato.